What is the Definition of 'Quasi-Drugs' under the 'Japanese Pharmaceutical and Medical Device Act'? Understanding the Efficacy and Effects that Can Be Expressed in Advertisements
For manufacturers and retailers such as drugstores dealing with quasi-drugs, conducting checks in accordance with the Japanese Pharmaceutical Affairs Law (薬機法) is a routine part of risk management. However, the standards for acceptable advertising under the Pharmaceutical Affairs Law can be ambiguous, making it difficult to make judgments, and there are cases where violations occur unintentionally.
In this article, our attorneys will explain the appropriate expressions for efficacy and effects required at the application and advertising stages for quasi-drugs, in order to prevent unintentional violations of the Pharmaceutical Affairs Law.
What are Quasi-Drugs under the Japanese Pharmaceuticals and Medical Devices Act?
The Pharmaceuticals and Medical Devices Act (Act on Securing Quality, Efficacy and Safety of Products Including Pharmaceuticals and Medical Devices) Article 2, Paragraph 2 defines “Quasi-Drugs” as follows:
“Quasi-Drugs” in this Act refers to the following items that have a mild effect on the human body:
1. Items used for the purposes listed from (a) to (c) below (excluding items used for the purposes defined in the previous paragraph, item 2 or 3, in addition to these purposes), which are not mechanical devices or similar:
(a) Prevention of nausea and other discomforts or bad breath or body odor
(b) Prevention of heat rash, chafing, etc.
(c) Prevention of hair loss, hair growth or hair removal
2. Items used for the purpose of exterminating rats, flies, mosquitoes, fleas and other similar organisms for the health of humans or animals (excluding items used for the purposes defined in the previous paragraph, item 2 or 3, in addition to this purpose), which are not mechanical devices or similar
3. Items used for the purposes defined in the previous paragraph, item 2 or 3 (excluding items listed in the previous two items), which are designated by the Minister of Health, Labour and Welfare
In other words, “Quasi-Drugs” are products that have a milder effect on the human body compared to “Pharmaceuticals” and are positioned between “Pharmaceuticals” and “Cosmetics”. Examples of “Quasi-Drugs” include medicated cosmetics, nutritional drinks, hair dyes, medicated toothpaste, antiperspirant sprays, hair growth products, and insecticides.
Furthermore, products that were transitioned from “Pharmaceuticals” to “Quasi-Drugs” due to the amendment of the “Pharmaceutical Affairs Law Enforcement Order” in Heisei 11 (1999) are called “Newly Designated Quasi-Drugs”, and those transitioned due to the amendment in Heisei 16 (2004) are called “New Range Quasi-Drugs”. These fall under the “Designated Quasi-Drugs” defined in Article 2, Paragraph 2, Item 3 of the Pharmaceuticals and Medical Devices Act.
“Quasi-Drugs” contain effective ingredients for the efficacy and effects designated and approved by the Ministry of Health, Labour and Welfare at a certain concentration, and are mainly created for the purpose of “prevention” rather than “treatment” of diseases. Also, the term “medicated” is only allowed for “Quasi-Drugs”, which have a milder effect than “Pharmaceuticals”, so “medicated = Quasi-Drugs”.
The Pharmaceuticals and Medical Devices Agency (PMDA) explains the definition and personalityistics of “Quasi-Drugs” as follows:
1. The effect on the human body is mild. (Pharmaceuticals and Medical Devices Act Article 2, Paragraph 2)
2. It is not a pharmaceutical. (Pharmaceuticals and Medical Devices Act Article 2, Paragraph 1 and 2, Director’s Notice No. 464 of the Pharmaceutical Affairs Bureau, September 6, Showa 37, etc.)
① Items that exceed the range as a Quasi-Drug in terms of ingredients and quantity or essence are treated as pharmaceuticals, regardless of their efficacy or effects.
② Even if the ingredients and quantity or essence are within the range allowed as a Quasi-Drug, items that deviate from the range as a Quasi-Drug in terms of efficacy or effects or usage and dosage are treated as pharmaceuticals.
3. The item is not one of the following. (Director’s Notice No. 464 of the Pharmaceutical Affairs Bureau, September 6, Showa 37)
a. Items equivalent to poisonous or deleterious substances
b. Pure substances
4. The main purpose of use is “prevention”. (Director’s Notice No. 464 of the Pharmaceutical Affairs Bureau, September 6, Showa 37, etc.)
※ For Newly Designated Quasi-Drugs and New Range Quasi-Drugs, refer to the relevant notices, etc.
5. It is a product that is expected to be used continuously in daily life.
6. In principle, it is not intended to be used under the management of medical professionals such as doctors and pharmacists.
“Basic Points to Note When Applying for Approval of Quasi-Drugs (2021 Edition)”[ja]
For more detailed explanations on the classification criteria for pharmaceuticals and other items under the Pharmaceuticals and Medical Devices Act, please refer to the following article.
Reference Article: “What is the Pharmaceuticals and Medical Devices Act (former Pharmaceutical Affairs Law)? Explanation of Purpose, Regulated Items, and Advertising Regulations”[ja]
About the Efficacy and Effects that can be Expressed in “Quasi-Drugs”
In the case of “Quasi-Drugs” (Japanese Quasi-Drugs), the range of efficacy and effects that can be displayed is determined by the Ministry of Health, Labour and Welfare according to the type of quasi-drug. Advertising that deviates from this range will be in violation of the Pharmaceutical Affairs Law (Japanese Pharmaceutical Affairs Law).
Different Expressions of Efficacy and Effects in General Cosmetics and Medicated Cosmetics
As mentioned earlier, medicated cosmetics, which are quasi-drugs, can claim not only the efficacy and effects of the specified active ingredients, but also the 56 efficacy and effects that can be displayed in general cosmetics. Therefore, they can conduct more persuasive advertising than general cosmetics.
【Differences between General Cosmetics and Quasi-drugs】
| General Cosmetics | Medicated Cosmetics | |
| Range of Efficacy and Effects | 56 Efficacies Designated by the Ministry of Health, Labour and Welfare | Approved Efficacy + Efficacy of General Cosmetics |
| Inclusion of Active Ingredients | None | Included |
There are three points to note when medicated cosmetics claim the same efficacy and effects as general cosmetics (56 efficacy and effects designated by the Ministry of Health, Labour and Welfare):
- Do not give the misunderstanding that it is a cosmetic that conceals the original purpose of the quasi-drug.
- Ensure that it is not something that could pose a health and hygiene problem when used for cosmetic purposes or methods (such as shampoo or medicated soap containing disinfectants).
- Do not give the misunderstanding that the efficacy and effects have been approved as those of the quasi-drug.
For detailed criteria on whether the efficacy and effects and expressions in advertisements of quasi-drugs, including medicated cosmetics, violate the Pharmaceutical and Medical Device Act, please refer to the Ministry of Health, Labour and Welfare’s “Explanation and Notes on the Standards for Proper Advertising of Pharmaceuticals, etc.”[ja] and the Japan Cosmetic Industry Association’s “Guidelines for Proper Advertising of Cosmetics, etc.”[ja].
Examples of Expressions that Violate the Japanese Pharmaceuticals and Medical Devices Act
The term “anti-aging” is well-known as a keyword that may violate the Japanese Pharmaceuticals and Medical Devices Act and regulations related to medical advertising.
Anti-aging (English: Anti-Aging) refers to “prevention and healing of symptoms associated with aging, anti-aging, and anti-senescence”.
The Ministry of Health, Labour and Welfare (MHLW) has expressed the following view on the term “anti-aging” in the “Medical Advertising Guidelines”[ja] (published on May 8, Heisei 30 (2018)).
Anti-aging clinics or (simply) anti-aging are not recognized as medical department names, nor are they the content of medical treatment with approved drugs and medical devices under the Pharmaceuticals and Medical Devices Act, and they are not allowed as advertisements.
Also, in the “Summary of the 4th Medical and Nursing Care Working Group Meeting”[ja] held in December of Heisei 30 (2018), the following description is given (the expression has been changed from the original text to match the content).
“The Ministry of Health, Labour and Welfare does not uniformly say that all expressions of ‘anti-aging’ are bad, but it is difficult to accept those that simply attract by saying ‘anti-aging’. For those that meet the conditions, they are treated as information that can be provided even for things other than ‘advertisable matters’.”
Based on the above, the use of the term “anti-aging” in medical advertising is basically not allowed, but it may be exceptionally allowed if it meets the requirements for lifting restrictions on the Medical Advertising Guidelines and is intended to explain the content of medical treatment. Also, “anti-aging” is an expression that claims the efficacy of pharmaceuticals, so it cannot be used in advertisements for quasi-drugs.
On the other hand, similar words such as “aging care” and “aging countermeasures” are not expressions that represent medical acts and pharmaceutical efficacy like “anti-aging”. Therefore, they may be posted on quasi-drugs within the range of approved efficacy.
“Aging care” refers to care with cosmetics, etc. according to age. In advertisements for quasi-drugs, the expression “aging care” cannot be used for the purpose of claiming “treatment” or “improvement” of diseases classified as pharmaceutical efficacy. It can only be used if it can be interpreted as meaning “care for skin condition according to age, which is recognized as the efficacy of cosmetics, etc.” considering the context before and after.
Thus, while “anti-aging” is an expression that means medical acts and pharmaceutical efficacy, “aging care” is an expression that means “care according to the current state”, so it can be said that it has high compatibility with cosmetics and quasi-drugs.
Also, expressions such as “whitening” and “whitening” may be used if they follow certain rules, such as using them with makeup effects and restrictions.
For specific examples of each expression allowed under the regulations of the Pharmaceuticals and Medical Devices Act, please refer to the “Guidelines for Proper Advertising of Cosmetics, etc.”[ja].
Reference article: Points to Note about Advertising Expressions for Cosmetics and Health Foods[ja]
Points to Consider in the Manufacturing and Sales of Quasi-Drugs

When manufacturing and selling quasi-drugs, it is necessary to obtain permission or approval from the Minister of Health, Labour and Welfare or the governor of each prefecture. In this article, we will explain the basic points to consider when obtaining the necessary permissions and approvals in the distribution process of quasi-drugs.
Firstly, in order to manufacture and sell (ship) quasi-drugs, it is necessary to obtain a manufacturing license and a manufacturing and sales license from the governor of each prefecture (Japanese Pharmaceutical Affairs Law Article 12 and 13, Pharmaceutical Affairs Law Enforcement Order Article 80, Paragraph 2). If you are manufacturing quasi-drugs overseas, you need to obtain foreign manufacturer certification from the Minister of Health, Labour and Welfare (Japanese Pharmaceutical Affairs Law Article 13-3).
Also, when actually manufacturing and selling quasi-drugs, you must submit a manufacturing and sales approval application to the regulatory authority (the Minister of Health, Labour and Welfare or the governor of each prefecture) for each product, and obtain approval for safety, efficacy, and quality (Japanese Pharmaceutical Affairs Law Article 14).
The various permission applications mentioned above are made to the governor of the prefecture where the manufacturing plant or manufacturing and sales office is located. On the other hand, the approval application for each product can be made to either the Minister of Health, Labour and Welfare or the governor of each prefecture (for quasi-drugs other than medicated cosmetics, some of the approval authority has been delegated to the governors of each prefecture).
The application category for “quasi-drugs” is divided into 11 according to the ingredients, quantity, efficacy, effects, etc., and the types of required attachments also vary, so caution is necessary.
For more details, please refer to the “Procedures for Manufacturing and Selling Quasi-Drugs” published by the Pharmaceuticals and Medical Devices Agency (PMDA) here.
Please refer to the following diagram for the flow of each permission and approval application.
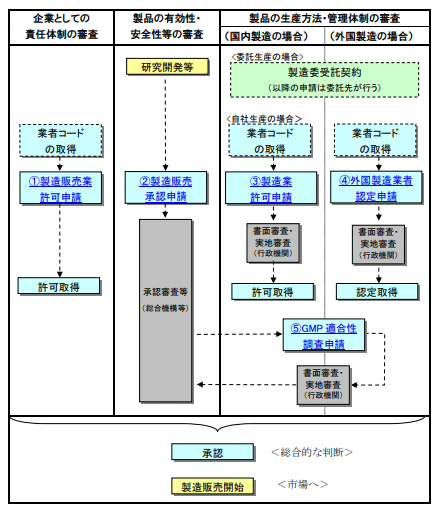
“Procedures for Manufacturing and Selling Quasi-Drugs”[ja]
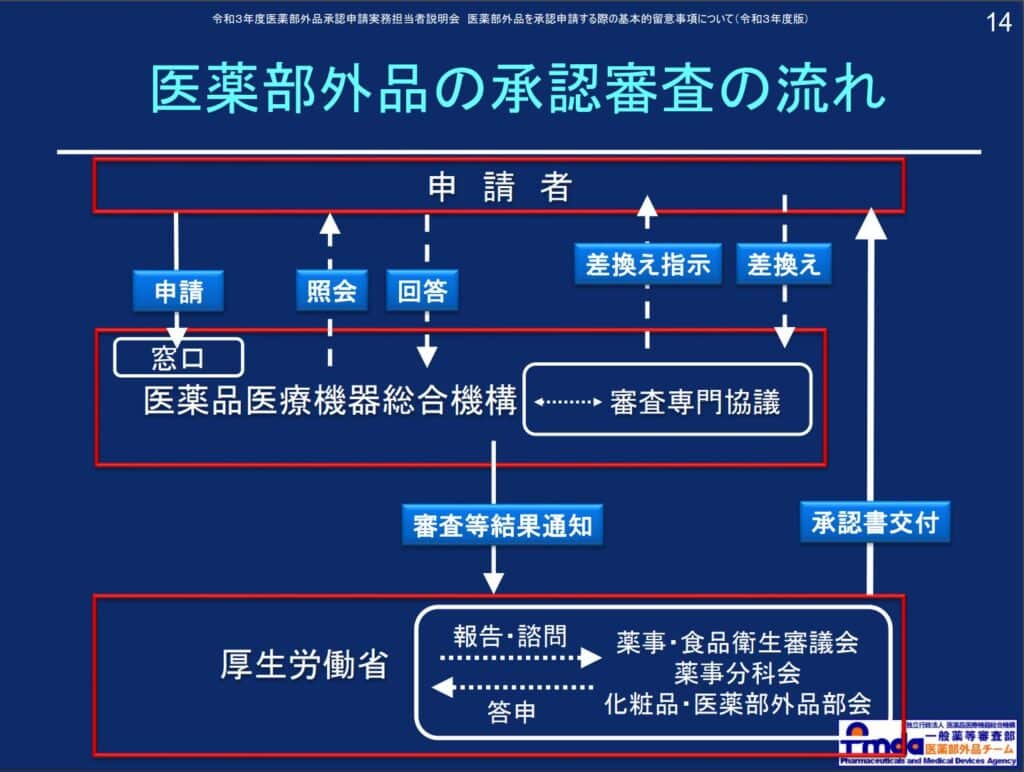
“Basic Points to Consider When Applying for Approval of Quasi-Drugs (2021 Edition)”[ja]
For the active ingredients that can be included in “medicated cosmetics” and “quasi-drugs”, please refer to the list of active ingredients in so-called medicated cosmetics published by the Ministry of Health, Labour and Welfare here[ja].
Also, there are 140 types of chemical substances selected as designated display ingredients (notified ingredients) that are mandatory for “quasi-drugs”. For more details, please see “Ingredients of Quasi-Drugs and Cosmetics Designated by the Minister of Health, Labour and Welfare”[ja].
Conclusion: For Legal Checks on the Japanese Pharmaceutical Affairs Law, Consult a Lawyer
With the amendment of the Japanese Pharmaceutical Affairs Law in Reiwa 1 (2019), an administrative penalty system was established for violations of advertising regulations for pharmaceuticals and other related products. This has led to stricter penalties for violations of these advertising regulations. The guidelines for advertising pharmaceuticals and other related products are revised annually, and those involved in the operation and consideration of such advertising, including businesses and advertising agencies, are required to respond carefully.
Interpreting the Japanese Pharmaceutical Affairs Law and determining whether expressions used in advertising are appropriate can be difficult to judge on your own. If you have any concerns, we recommend consulting a lawyer who is knowledgeable about legal checks on the Japanese Pharmaceutical Affairs Law.
Legal checks on the Japanese Pharmaceutical Affairs Law and other related laws, as well as proposing revised expressions, are highly specialized areas. Monolis Law Firm has formed a team specializing in the Japanese Pharmaceutical Affairs Law, and we handle article checks for a wide range of products, from supplements to pharmaceuticals.
Introduction to Our Firm’s Measures
Monolith Law Office is a legal office with extensive experience in both IT, particularly the internet, and law. We provide services such as legal checks of articles and landing pages, creation of guidelines, and sampling checks for various entities including media operators, review site operators, advertising agencies, Direct-to-Consumer (D2C) businesses such as supplements, cosmetic manufacturers, clinics, and Application Service Provider (ASP) businesses. Details are provided in the article below.
Areas of expertise at Monolith Law Office: Article and Landing Page Checks for Pharmaceutical and Medical Device Law, etc.[ja]
Category: General Corporate